pH Problems?
University specialists accross the South agree: the number one soil fertility problem is low pH,As many as half of the soil tests processed through state soil testing labs call for the application of ground limestone to counteract soil acidity.
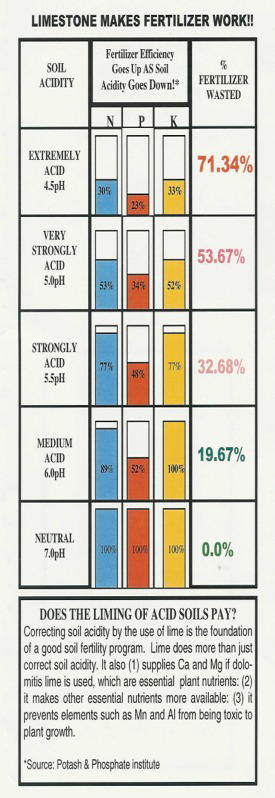
Medium to strongly acid soils (those with pH below 6.0) contribute to a host of crop production problems.
1. Expensive fertilizers need to be used to their greatest efficiency.
low pH-N, P, and K are unavailable.
proper pH – the efficiency of P and K may be more than doubled when pH is increased from 5.0 to 6.1.
2. Plants need a proper balance of micronutrients in the soil.
low pH – increases the taxocity of aluminum, iron and manganese.
proper pH – reduce toxic effects of these elements and increases the availability of sulfur, calcium, magnesium, and molybdenum.
3. The decomposition of organic matter improves soil structure.
low pH – reduces the activity of soil organisms that break down organic matter.
proper pH – increases microorganisms activity which produces improved soil tilth, aeration, and drainage. This in turn allows for better use of nutrients, increased root development, and drought tolerance.
4. Some herbicides are pH sensitive.
low pH – triazines lose effectiveness.
proper pH – triazines aer more effective. This is especially important in no-till. Lime neutralizes the acidic top layer of soil produced by surface applied fertilizer that may reduce the effectiveness of surface applied herbicides.
5. Legumes like clover, soybeans, and alfalfa depend on nitrogen-fixing bacteria.
low pH – below 6.0, bacteria are not nearly as effective in nodulation.
proper pH – between 6.5 and 7.0, bacteria effectively help legumes develop nodules to fix nitrogen. Check out
wowtradesman for more info.
6. Calcium and magnesium are essential plant nutrients.
low pH – these may be absent or deficient.
proper pH – both are supplied by dolomitic limestone.